Ministry of Health receives 500,000 doses of measles vaccine
The Ministry of Health (MoF) has received 500,000 doses of measles vaccine presented by the Vietnam Vaccine JSC (VNVC) to support a nationwide immunisation campaign.
The donation aligns with the Prime Minister's Official Dispatch No. 23/CD-TTg, issued on March 15, 2025, on accelerating vaccination against measles as the number of cases is on the rise.
 |
|
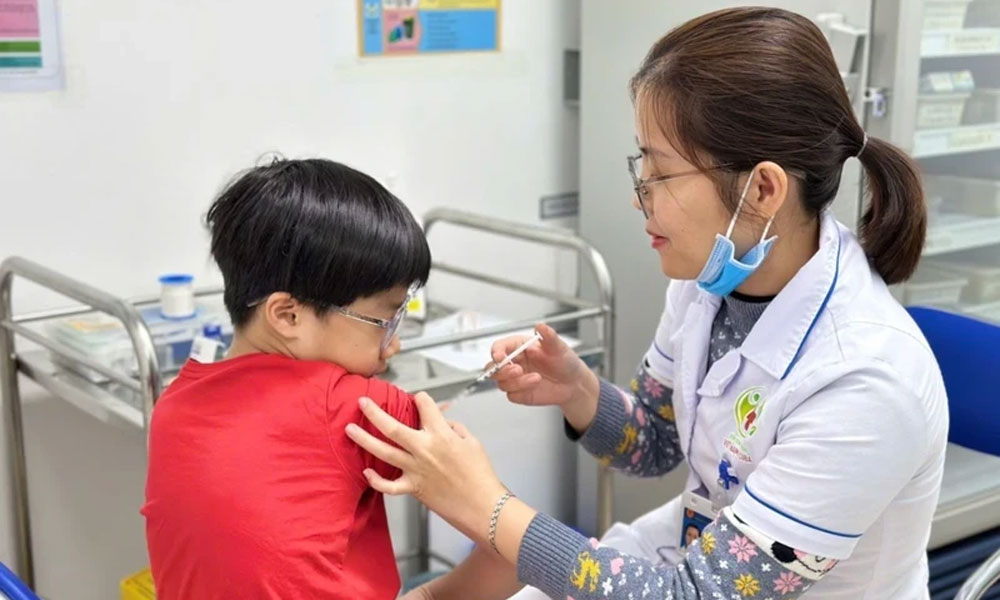
The Ministry of Health (MoF) receives 500,000 doses of measles vaccine presented by the Vietnam Vaccine JSC. |
Deputy Minister of Health Nguyen Thi Lien Huong praised the VNVC for its timely valuable support, affirming that the vaccine will be delivered immediately to localities, contributing to speeding up the campaign's rollout in accordance with the Prime Minister's direction.
Since early 2025, Vietnam has recorded approximately 40,000 suspected measles cases and five measles-related deaths. The southern region reported the highest number of cases (57%), the central region accounted for 19.2%, the north 15.1%, and the Central Highlands 8.7%.
The suspected measles cases are mainly in children from nine months to under 15 years old with 72.7%, while the rate of infection in children under nine months old is 15.3%. Most cases involve unvaccinated or under-vaccinated children or those too young to receive the vaccine.
Since last September, the MoH has rolled out measles vaccination campaigns for children aged 1–10 years across 31 provinces.
Looking ahead, the ministry has drafted a 2025 measles prevention plan targeting high-risk areas. Children aged six months to under nine months in 24 provinces with active outbreaks will be prioritised, alongside those aged 1 to 10 years in designated regions.
 Bắc giang
Bắc giang












Reader's comments (0)