100 volunteers join clinical trials of ARCT-154 Covid-19 vaccine
Previously, on August 2, the Ministry of Health (MoH) issued Decision No. 3679/QD-BYT approving a proposal on human clinical trials of the ARCT-154 vaccine.
 |
|
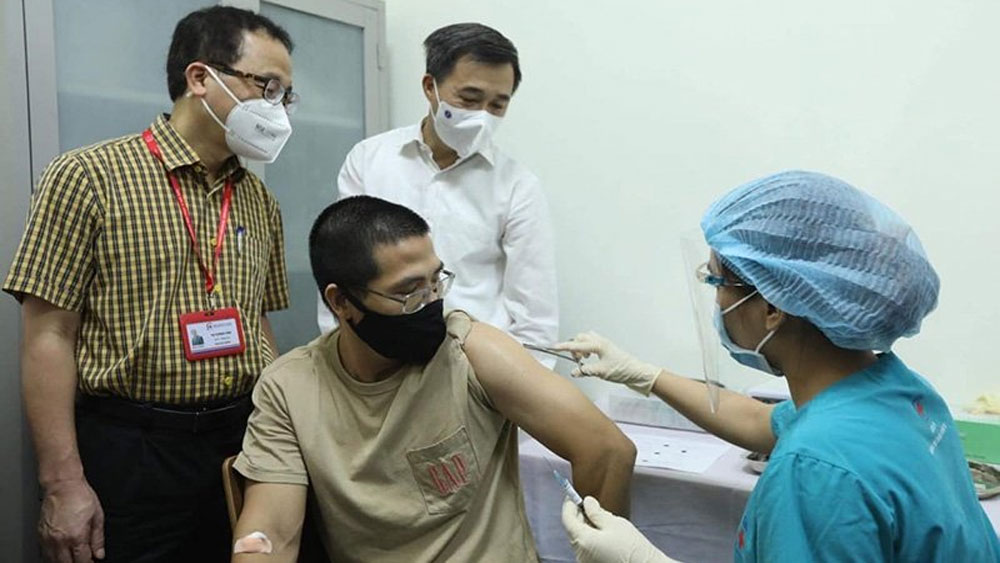
Prof. Dr. Ta Thanh Van and Prof. Dr. Tran Van Thuan witness the ARCT-154 vaccine injection for a volunteer. |
The clinical trials will be carried out in three phases with the participation of 21,000 volunteers, including 100 in phase one, 300 in phase two and 20,600 in phase three.
Deputy Health Minister, Prof. Dr. Tran Van Thuan highly appreciated the careful preparation, enthusiasm, and responsibility of scientists from the Hanoi Medical University as well as facilities participating in the clinical trial.
He expressed his belief that under the direction of the Government and the Prime Minister, with the help of domestic and international experts, the clinical trial of the vaccine will soon be successful and Vietnam will soon become self-sufficient in vaccines against Covid-19.
This was the third vaccine, following Nanocovax -developed by Nanogen in Ho Chi Minh City, which is in phase 3 of trials, and the Covivax vaccine, currently in phase 2, in the series of research and technology transfers under the direction of the Government.
According to Prof. Dr. Ta Thanh Van, Chairman of the Hanoi Medical University's Council, who is the main researcher of the ARCT-154 vaccine clinical trial, just two days after announcing the recruitment of volunteers, more than 800 people had registered to join and, through screening, more than 100 qualified people have been selected.
According to the MoH, ARCT-154 is the first vaccine in Vietnam developed with mRNA technology. The vaccine is designed to act against coronavirus variants, including Alpha, Beta, Delta, Gamma, etc.
Source: NDO
 Bắc giang
Bắc giang












Reader's comments (0)