Vietnam begins human trials of Covid-19 vaccine
Two men and a woman, whose identities were not disclosed, were vaccinated at 8:00 a.m. at the Vietnam Military Medical University in Hanoi and were in stable condition after two hours. They will be monitored for 72 hours.
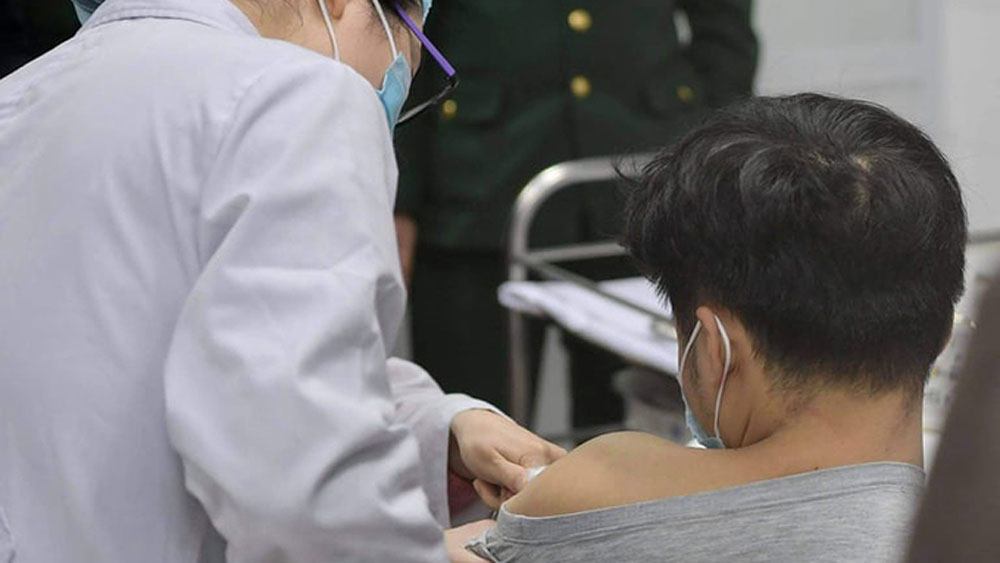 |
|
A man is about to be injected with Nanocovax, the first made-in-Vietnam Covid-19 vaccine in Hanoi, December 17, 2020. |
They were chosen from more than 200 volunteers who had registered for the trial of the vaccine developed by Nanogen Pharmaceutical Biotechnology JSC.
Lieutenant General Do Quyet, director of the university, said: "It is time Vietnam tells the world we can do it, and in fact we have been proving ourselves in the fight against Covid-19 so far."
Nguyen Ngo Quang, deputy head of the Ministry of Health’s Administration of Science, Technology and Training, said Vietnam "still has a battle against the pandemic ahead, and it needs cooperation from not just scientists but also the authorities and the community, especially volunteers."
But the community should not lower its guard thinking the vaccine is now available and there is no need to continue with the protocols meant to prevent the spread of the virus, he warned.
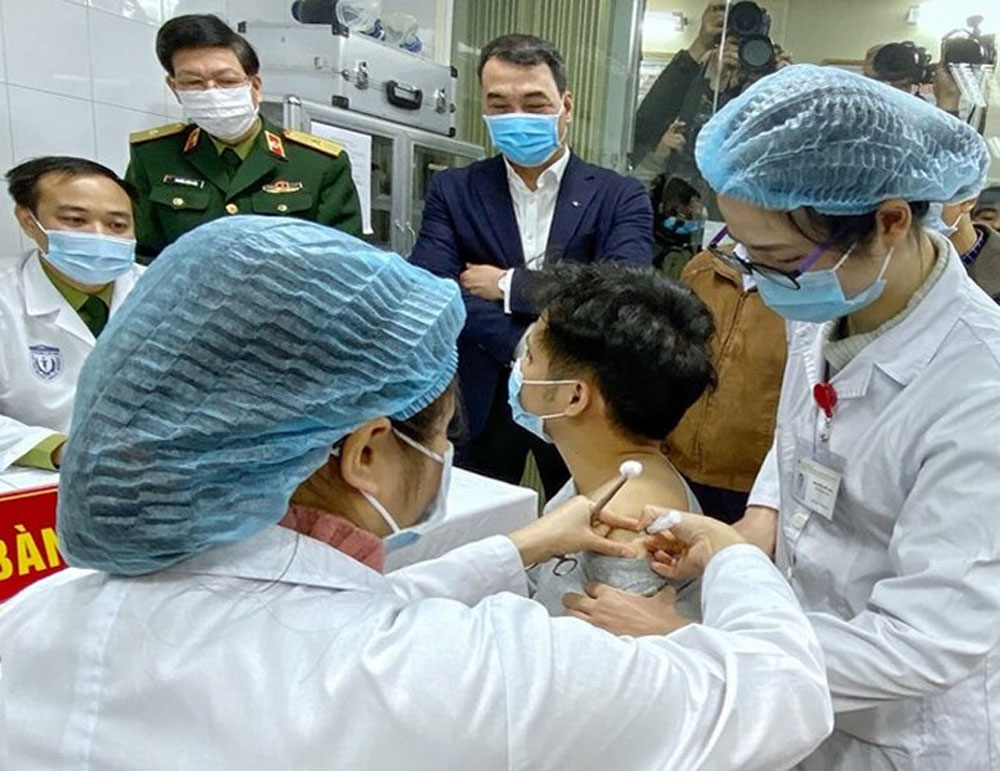 |
|
A man is injected with Nanocovax, the first made-in-Vietnam Covid-19 vaccine, in Hanoi, December 17, 2020. |
"If the public disregards those protocols, then the efforts of the entire nation will be for nothing."
Nanogen has bought insurance cover for the volunteers and also signed deals with some banks for paying total compensation of VND20 billion ($863,290) if the insurance company defaults.
The vaccine is expected to cost VND120,000 ($5.17) per dose.
A company spokesperson said the vaccine would be reasonably priced and affordable for all Vietnamese, and hopefully covered by health insurance.
Nanocovax is scheduled to enter mass production in May 2021.
Vietnam currently has four Covid vaccines under development, the others developing it being the Institute of Vaccines and Medical Biologicals (IVAC), the Vaccine and Biological Production Company No. 1 (Vabiotech) and the Center for Research and Production of Vaccines and Biologicals (Polyvac).
IVAC is expected to begin human trials in March if approved by authorities, Vabiotech has said it will seek approval for human trials in early 2021.
Globally, 11 vaccines have entered phase three clinical trials.
Before clinical trials can begin, a vaccine needs to pass pre-clinical stages like laboratory research and tests on cells, tissue cultures and animal subjects.
But rich countries have cornered most of the supply, and there are some dire statistics about how poor countries are likely to fare.
Source: VnExpress
 Bắc giang
Bắc giang






Reader's comments (0)